GEOROC have recently (July, 2005) made a huge effort at recording the analysed compositions of common rock forming minerals. I believe we actually began the practice of combining rock and mineral analyses at the Universitie de Montreal, in the 1978 era, when we would do the bulk rocks by XRF and Dr Ramon Coy-ll would do the minerals by probe. We were much helped by Art Chodos at Caltech who gave us standard olivines, garnets and pyroxenes. We also routinely did, eg Sr, Rb, on plagioclase separates from anorthosites, but not unfortunately, Zr.
Since then thousands of minerals have been analysed world wide, not always with happy results. Very few trace elements have been done, eg for about 5000 analysed plagioclases, almost none have been done for Sr, Zr. Even in the pyroxene group, the same situation prevails as in whole rocks, and about 2000 have been done for a minor number of TE elements but not including the ME, Ca,Mg and Fe.
Deer, Howie and Zussman in their well-known textbook give mineral compositions for a dozen or samples each but no accurate trace elements, for which we still have inadequate data. A great deal remains unknown, we know even less about the compositions of minerals than we do about some rocks, the “random samples, analysed for random elements by random methods” approach having once again fallen down.
It does not seem that anyone has yet showed the compositions of large numbers of even the common rock-forming minerals, so we show some diagrams here. It is all very incomplete due to lack of ICPMS trace data, yet a few people, eg Norman & Garcia.(1997) on Hawaiian 1955 pyroxenes, have shown it can be done.
Pyroxenes
There are currently about 17,000 lines of data, a big advance on the few hundred we had accumulated in “GEOKEM”. Of these about 14,100 include Ca, Mg and Fe. It is well known that clinopyroxenes range from magnesian diopside through augites and sub-calic augites to iron-rich hedenbergites, but the range as seen in all rock types from mantle xenoliths to phonolites - rhyolites is extreme and shows why partition coefficients derived from whole rock/crystal ratios in a range of rocks are so different as to be meaningless. In general there is a tendency for clinopyroxenes from peridotite xenoliths to be extremely LILE depleted, but in AOBs and phonolites they can be quite enriched. La/Sm can range from <0.5 to as high as 20. Norman & Garcia, (1997) made one of the few complete studies on the TE of pyroxenes from the 1955 Kilauea eruption and found Nd considerably in excess of Ce, with a La/Sm ratio of about 0.3
Again we have poor naming by some authors. Minerals with <0.04% CaO named as either "clinopyroxene" or "augite" for example are not likely to be correct. About 50 olivines are included as “orthopyroxene”. No clinopyroxenes are identified as “pigeonite” though several hundred have a CaO elevated above normal orthopyroxene. A few are termed “aegirine” though others usually from an alkaline association have soda up to 12.5% but ae not so named. Many are simply labeled “pyroxene” even though some are definitely no such thing.
As time permits we will look at pyroxenes from ORBs, OIBs, and Arc rocks to see whether there is any consistent difference, but none is visible so far.
 |
Variation diagram for all pyroxenes. Note wide range in Na, Ti, Ca as well as Mg/Fe. Both magnesian orthopyroxenes and clinopyroxenes become more aluminous with increasing iron. Sr may range from almost zero to as high as 1000 ppm in aegirines. |
| |__| |
FeOT / MgO Notice the parallelism between olivines on the right, orthopyroxenes and less constant clinopyroxenes. |
Clinopyroxenes
 |
Ternary CaO-MgO-FeO diagram for more than 18,300 pyroxenes. This diagram shows wt% oxide, not mol% as is the common practice, so CaO appears elevated in the high Mg members of lower sg. This will be recalculated at some point in the future, but would make a good class exercise. There is an apparent gap between orthopyroxenes at the base and pigeonites with about 10% CaO.
Sub-calcic augites are often metastable and found in any lava that has been rapidly chilled. Clinopyroxenes also show a wide range in trace elements. Cpx in mantle xenoliths found in phonolites from East China may have x50 chondrite levels of LREE (~ 18ppm) but may be sub 1ppm in mantle cpx that have been partially melted. (Jianping Zhang, 2005, Geoch.Cos.Act. 60, 3401-3418). Clinopyroxenes appear to be the main mantle reservoir of the LILE. |
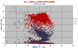 |
All ORB Clinopyroxenes variation diagram.
Note the TiO2 increasing steeply with declining MgO. Cpx composition varies widely with the composition of the magma it is formed in, both Al2O3 and TiO2 reaching very high levels in alkaline rocks. |
Orthopyroxenes and Pigeonites
An indistinct gap in CaO is seen between orthorhombic orthopyroxene and triclinic pigeonite. Pigeonite may occur in the groundmass of tholeiitic basalts and in CFBs mantling the OPX phenocrysts. In CFB sills, the pigeonite is often inverted to an iron-rich Opx, with exsolved blebs of clinopyroxene scattered throughout. Neither pigeonite nor orthopyroxene occurs in alkaline ankaramites.
Ortho-pyroxenes form a series between the magnesian end-member enstatite (MgSiO3) and the seldom-seen pure iron ferrosilite, FeSiO3). Small amounts of Al2O3 and CaO may be present but surprisingly little data exists on substitution by Mn, Co, Ni, Cr, Zn or other metals.
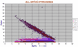 |
Variation diagram for all orthopyroxenes, ranging from 37% MgO ( = En 95 (mol %), to about En 50 but with extemely fractionated members extending to En 23. The alumina content may reach 8% in magnesian members. Samples with 4-5% CaO (= 10% Wo (mol%)) are pigeonites though not so named. Wollastonite is the Ca pyroxene, CaSiO3, usually found pure only in contact metamorphc rocks.
Large (1, 2cm) orthopyroxenes may occur in silica saturated CFB sills and gabbroic intrusions. Enstatite is found in very oxidised chondrite meteorites and in diogenites. Opx is also commonly found in Harzburgite peridotites and as a groundmass mineral in some tholeiitic olivine basalts. |
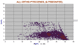 |
All orthopyroxenes showing detail of CaO enrichment. All are termed "orthopyroxene" but we assume the high CaO members are pigeonites. Some clarification seems to be needed here.
|
Micas
Some 1400 mica analyses of the sheet silicates show some remarkable features. The bulk of them for a single series from 0 - 26% MgO with FeOT declining from about 35% to about zero. K2O remains more or less constant at 6-10% and alumina at about 12 - 18%. Notice that the TiO2 tails off parallel to FeOT at high MgO contents.
The high MgO end-members are the phlogopites, as found in mantle rocks but also in lamprophyres, and pass continuously into biotites. A few high CaO margarite “hard micas” are included. Very few muscovites seems to have ever been analysed; they are distinguished by their low FeO and MgO. A small group of high alumina (35%) sericites are included. Sericite is usually a micaceous alteration product of potassium feldspar.
In spite of the fact that in coarser grained rocks micas are easy to separate, there is disappointingly little TE data. Of 30 REE the majority have a La/Sm of about 6.
A similar number of Zr/Nb seem to show a very low Zr/Nb ratio of about 0.4 - 1. Again this could explain the Nb-Ta depletion in the rocks of subducted margins, but there are far too few data. In phlogopites Nb seems to always exceed Zr and to have a range in K2O the same as in common biotites. TiO2 seems to be highly variable, we do not know as yet why. The LREE seem to be very low, but we do not have as yet enough data.
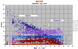 |
Variation diagram for 1400 micas, mainly phlogopites to biotites with minor celadonite, sericite, muscovite and margarite. |
Amphiboles
The amphiboles, ranging from common hornblendes as seen so often in Arc andesites to the reddish oxy-hornblendes and kaersutites of alkaline differentiates tend to reflect the composition of the rocks in which they occur. A range of 0 - ~30% MgO is present.
Of 2658 lines of data, only ~ 100 have been done for Zr and 67 for Nb. The range for Zr is 43 - 500 and may be much greater. The overall trend is for a very low Zr/Nb ratio of about 2, but no data at all is available for the hornblendes of calc-alkaline rocks, not even from the common amphibolite blocks so often seen in andesite flows.
Possibly hornblendes are the sink for the Nb-Ta depletion seen in all continental rocks, but again there is too little data to make positive statements.
 |
Variation diagram for 2650 amphiboles of all origins. The general range is from 0 - 30% MgO.
The high FeOT samples in the 15 - 30% MgO range are cummingtonites. Some with 15 - 22% MgO and high CaO, (15 - 22%) are either from komatiite spinifex, or from Tahitian ankaramites.
Again the lack of TE data means that detailed description is not possible as yet. |
Plagioclases
Plagioclases are the most common of all rock-forming minerals. As is well known they range in a complete solid solution series from albite (Na, Al,Si3O8) to anorthite (Ca,Al2,SiO6). The most common composition seen in basalts is labradorite (An50 - An70); in andesites it is usually andesine (An30-50). In dacites the feldspar may be oligoclase (An30-10) and albite (An(10-An0) in granites - rhyolites. Pure anorthite is seldom found.
Variable amounts of Sr, Ba, Rb, Ga, may be included within a feldspar, but of about 5000 lines of data, not enough TE are included as yet to be definitive,. In the Anorthosites of Quebec we found about 60 to 400 ppm Sr, with Sr increasing in the more sodic members.
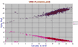 |
CaO / Al2O3, Na2O for 2118 ORB plagioclases. Data are not yet available for other rock series. Most feldspars occur between An 92 (mol%) and An 53, but a few almost pure anorthites are found and some groundmass albites, (or else albite jacketing more calcic feldspar in highly fractionated ORBs), may be down to An 7. In intrusive gabbroic bodies where the first formed plagioclase may be very calcic, if the feldspar is continuously removed from reaction with the residual basaltic liquid, can result in a wide range of feldspar composition within a single intrusion. |
Garnets
While common in metamorphic rocks, garnets are not found in normal igneous rock series. However they do occur in mafic xenoliths and inclusions as sometimes found in alkaline flows. It is assumed that they form only under very high pressure in the lower mantle. Garnets have the general composition of X3,Y2,(SiO4)3 where X may be Mn, Mg, Fe or Ca; Y may be Al, Cr, Fe+++ or V+++. Many other elements may substitute including the heavy REE but again there is as yet surprisingly little data.
 |
Variation diagram for 300 garnets. It is common practice to describe garnets as a percentage of the assumed end-members; pyrope being the magnesian endmember, grossular is the calcic garnet; andradite the iron-rich one and spessartite for the Mn-rich. |
However, we see that pyrope forms a series with the common almandine, but while about 6-7% CaO is included, it does not project towards the high CaO (~33%) grossular. Andradite has very low alumina (<4%), very high TiO2, (7 - 14%) and lowish iron (18 - 24%)
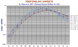 |
An unusual garnet series from xenoliths in the Yingfengling volcano on the Leizhou Peninsular of South China (opposite Hainan Island). The REE show absolutely no correlation of La/Sm, La/Lu, or Y/Yb or Zr/Nb but are well ordered nevertheless. Involvement of this garnet with magma production in the mantle should produce unusal REE patterns in the melt. |
Olivines
Olivines as is well known form a complete solid solution series between the magnesian end-member forsterite (Mg2,SiO4) and fayalite (Fe2,SiO4). Olivine makes up about 70 -90% of the Earth's mantle (or more) and may make up 20% of picritic lavas of the Hawaiian Islands and Reunion. Elsewhere in both tholeiitic and alkaline rocks it is a minor constituent in basalts.
PETDB have recently posted 3012 analysed olivines for ORBs, mainly for ME only. These give a range of Fo 93 to Fo 75 with a few groundmass olivines extending to Fo65. Olivines in the Hawaiian shields average Fo 85 ( see Hawaiian chapter). No distinction has been made for olivines from EMORB as opposed to NMORB but NMORBs of 10% MgO usually include olivines of about Fo 88 - Fo90.
Other divalent metals including Ni, Co, Zn, Mn substitute for Mg-Fe usually in the range 1000 - 2500 ppm Ni, 80 - 100 ppm Co, 100 - 120 Zn and about 0.18% MnO, but again there is little data. NiO determined by EMP is of low accuracy and appears to suggest that no Ni is included below Fo30 - Fo35, which is not likely.
Whether the ratio Mg/Ni remains more or less constant over a wide range of olivine composition is simply not known as yet. Ni contents of over 3000 ppm may occur in very magnesian Archaean komatiites but the authors of this data refused to confirm it.
GEOROC intends to add data on all published olivines, which may included more trace data. (July, 2005).
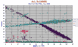 |
Variation diagram for 7,500 world-wide olivines. Notice increase increase in manganese with FeO. Obviously some other misnamed minerals are present: what are they? The olivines range from about Fo1 to Fo 95, probably the simplest mineral known as well as the most common in the whole solar system. |
 |
NiO in olivines. Data is of low accuracy but the distribution indicates that olivines of less than Fo 35 may have little or no Ni, with a maximum of about 4000 ppm in komatiites or dunites. |
Spinels
Spinels are usually seen as quite tiny cubic minerals in high temperature rocks, in fact in basic lavas chromian spinels may be the first minerals to form, though in very small quantity. In high pressure mantle xenoliths, almost all the alumina present may be in the form of spinel with plagioclase absent. GEOROC have now assembled about 5000 analyses of them, many more than the few hundred we had in the GEOKEM database and the compositional range is much greater than popularly supposed. Spinel SS, has the composition MgAlO4 and forms a complete solid solution series with Hercynite FeAlO4. However, the trivalent Al+++ may be replaced by Cr+++ so as we shall see spinels may range from 70% Cr2O3 to 70% Al2O3 in low Ti variants.
Fe+++ may also replace Al+++ leading to magnetite as may Fe++ and Ti++++ leading to the titanomagnetites. A few samples are seen with an excess TiO2 being present at greater than 50% showing that some ilmenite has been mistaken for spinel, even the occasional rutile with in excess of 90% TiO2.
Zn and Mn are present at a <2% and < 5% levels showing that the zincian spinel Gahnite and the manganese spinel Galaxite often seen in metamorphosed ores are not present in common igneous rocks. In general high Ti spinels are low in Cr but a titanomagnetite with 20% TiO2 may still have 10% Cr.
To sort out which environment favour which extremes in spinel would be a major task and beyond our resources. Unfortunately while it is common for people studying volcanic samples to include a small number of EMP determinations for pyroxenes, spinels, micas, amphiboles etc, they are seldom done in the numbers required ( 200 – 1000) to positively type them. We will show a few examples and add more as they are found.
About 2/3 of all samples are from mantle xenoliths including those from the rejuvenation stage Hawaiian series, and from alkaline and calc-alkaline rocks. Spinels from dunites, wehrlites, harzburgites etc are invariably high Cr with very low Fe and Ti as are some from picrites of Kilauea where spinels may be trapped in porphyritic olivines. Spinels in basalts tend to have increasing iron, while those from andesites are mainly titanomagnetites and magnetites.
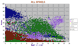 |
Variation diagram / MgO for 4600 spinels, data from a GEOROC compilation. As may be seen the high alumina spinels have minimal iron, (<15%). Titanomagnetites seem to not have more TiO2 than ~ 28% though a few ilmenites with ~ 50% are present. |
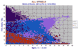 |
Barnes & Roeder have put together an immense database of spinels over many years. Here we see about 32,000 spinels from a wide range of crustal and mantle rocks. Al2O3 and Cr2O3 have the same cut-off point. This database is downloadable and can be found by Google. |
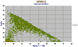 |
Al2O3 vs Cr2O3. The high Cr2O3 spinels are close to chromite (FeCr2O4) though some MgO, Fe+++, and Al+++ is always present. As seen below the high alumina spinels contain 10 – 20% of FeO and MgO. |
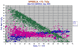 |
Al2O3 vs FeO, MgO and Cr2O3 for 1850 spinels with < 1% TiO2 |
| |__| |
Spinels from melilite melanephelinites (Bermudite) of Bermuda showing a range between magnetite and a chromian-magnesio-iron spinel. |
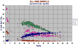 |
Spinels from ORBs. These do not have more than 45% Cr2O3. Those that do and approach the chromite end member in composition are found only in harzburgites, dunites, bonninites and komatiites. The gap between the chromian hercynites and the titano-magnetites and magnetites is filled by the spinels found in, among other rocks, the mela nephelinitic Bermudites. The high alumina, lower chrome minerals mainly appear in ultramafites, of what kind is not specified. |
This section is far from complete, it is intended to give an indication of the ME range in the common rock-forming minerals only. We also hope it will encourage more work on the trace element substitution ranges.