|
Isotopic Characteristics Of
Common Igneous Rocks
Introduction
It has long been claimed that as two isotopes of an element are identical in a chemical reaction, their ratios should remain constant in all geochemical processes. Any difference in isotopic ratio found between two igneous centres for example must have been inherited from the parental mantle material. This does not now appear to be entirely so.
Kamanetsky (2000, J.Pet. 41, (3) 411-430; and J.Pet. 43, 2002) has
shown in the partial melt series from Macquarie Island that a small
but significant increase in Sr 87/86 ratio takes place with decreasing
degree of partial melt, while the Nd.143/144 decreases. The Pb 206/204
and Pb 208/204 also increases with decreasing degree of melt.
In Oceanic basalts increasing alkalinity inferring a greater depth and pressure of magma generation, has a somewhat greater effect than the degree of melt. Basanites or alkali basalts have a higher 87/86 and lower Nd ratios than tholeiites. The most depleted (in LILE) tholeiites have the lowest Sr87/86 and highest Nd143/144 ratios.
Fractionation in Ridge tholeiites which leads to elevated Rb but constant Sr has only a very slight effect in raising the 87/86 ratio, but in more alkaline rocks, fractionation seems to have a very strong effect with phonolites, trachytes and pantellerites having extremely elevated Sr87/86 but at constant Nd143/144 though the latter differs between islands.
Small but significant differences in isotopic ratio are seen between different oceanic volcanic islands in addition to those due to difference in degree of melt, and to fractionation. Some islands may differ by factors of two three or four or even more in their relative K, Rb, La/Sm or Zr/Nb, yet have similar isotopic ratios. Others may not.
There seems to be a good deal we do not understand here!
 |
Sr/Nd diagram for partial melts from Macquarie. One point is somewhat fractionated. Smaller degrees of melt have the higher Sr 87/86 ratio.
Here we show the range of two of the three most commonly used isotopic ratios associated with geochemistry. Later the variation in lead ratios will be added. While there are now many thousands of isotopically analysed samples, the samples are often poorly identified which limits the interpretations we can make. |
Sr 87/86 and Nd 143/144 ratios seen in the Oceanic ridges:
 |
Isotopes of the East Pacific Rise. Basalts more depleted than Macquarie have Nd 143/144 higher than 0.5132, those near 0.513 are more alkaline. |
 |
Isotopes of the Galapagos Rise. A limited range with Sr87/87 mainly below 0.703. |
 |
Isotopes of the Atlantic. These include a large number of whole rocks and some data are now quite old. The wider spread of data is to be expected in these variable rocks, but the more extreme data departures may be due error or alteration, they seem too great for fractionation. (Some very high Nd (>0.533) are cumulate blocks.) |
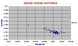 |
Isotopes of the Indian Ocean Rise.
As to be expected, rather variable, but indistinguishable from the MAR.
Other minor ridge centres such as the Juan de Fuca which have a variable range of chemistry also show a predictable scatter in isotopes. |
Isotopes of the OIB's
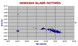 |
Isotopes of the Hawaiian Islands.
The high Nd ( > 0.5131) points include many ultramafic blocks, harzburgites, wehrlites, garnet websterites and pyroxenites. Rocks with less than 0.5128 Nd are alkali basalts, basanites and other post-shield rocks from Haleakala, late stage hawaiites from Mauna Kea, Hualalai etc. |
When time permits we may be able later to distinguish the different rock type in different colours etc.
The group of very low Nd 143/144 centred at 0.5122 are all from the HDSP Drill core from Mauna Kea. As Mauna Loa is indistinguishable from Kilauea such a difference seems unlikely. Other samples from the same laboratory show the same anomaly. It seems that the isotope lab concerned used a different base standard. If we can obtain the factor these will be corrected.
 |
Isotopes of Iceland.
Considering the wide range of rock type on Iceland, the isotopes are fairly compact. The high Nd samples are mainly the picrites of Theistareykir, and the low Nd ones from the alkaline centres. There seems to be only a small difference isotopically between rhyolites and basalts. Only those rhyolites of >70% SiO2 show a slightly elevated Sr87/87 of >0.7035 compared with the average basalt at 0.703 - 325 |
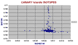 |
Isotopes of the Canary Islands.
While the basic alkali basalt, basanite, ankaramite is centred at about Nd 0.5129 as in other areas, the fractionates have above 0.71. These rocks are all phonolites, trachytes, and pantellerites. There is a general covariance with elevated Rb, and a general negative one with Sr, but no good relationship with Rb/Sr. The samples of low Sr, high Nd ratio are labelled "Coarse grained metagabbro xenoliths".
The alkaline Rivillagigedo Is west of Mexico show exactly the same elevation of Sr 87/86 in trachytes and pantellerites as does Ascension Id, though at slightly higher Nd 143/144, (>0.513), also occasional samples from the Cameroon Line centres on the West African coast. The Comoros Is in the Indian Ocean might also be expected to as well but no trachytes etc are included in the available data.
Ascension Id includes commendites, quartz trachytes and rocks called "rhyolites" but their isotopic difference with the "rhyolites" of Iceland is extreme, some of the Ascension "rhyolites" having a Sr 87/86 of 0.709. The Cape Verde Is might be expected to be similar to the Canaries but no trachytes are included, though the basic rocks are similar at 0.703 - 0.704. Here high Nd samples are labelled "nephelinite" and the low Nd ones "phonolite" but as few are analysed for major elements, presumably the classification is based on hand samples. |
| [____] |
Isotopes of the Tristan da Cunha Group.
These are quite different having a Sr 87/86 ratio of 0.705 and a low Nd ratio of about 0.5125. However, as this group along with Gough Id is well known for being more potassic, such a shift is not unexpected. The Rb for example is double that of the Canary Islands. We would expect also that Jan Mayen and Heard, possibly Kerguelen should also show a similar shift. However, Jan Mayen with exactly the same K/Na ratio have Sr 87/86 clustered at 0.7035. |
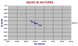 |
Heard Id in the southern Indian Ocean has an elevated average Sr 87/86 ratio of 0.755 and a Nd of 0.526. The sample on the extreme left is not analysed but is labelled "limestone". The point near 0.708 is a trachyte but then so are the points below 0.705. It is unusual to find trachytes with lower 87/86 than basanites etc, but more than one lineage is present on Heard, the rocks of Laurens peninsula being less alkaline. |
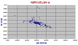 |
Kerguelen Isotopes.
These overlap with Heard Island as do samples from submarine Kerguelen Plateau. As Kerguelen includes a complete spectrum of rocks from tholeiites to basanites and trachytes to phonolites the data spread is to be expected. The point of lowest Nd 143/144 is unanalysed but labelled "Coarse-grained dunite". Presumably a peridotitic inclusion is meant. Such rocks can have a very alkaline interstitial matrix. The high Sr isotope samples are mainly "syenites" but some of even higher ratio, not shown, are simply labelled "Plutonic gabbro" but without any analyses. |
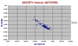 |
Society Islands.
Here we see a series similar to Heard Id but lying a little on the higher Nd ratio side and inclined at a steeper angle. Only six percent of 500 samples were analysed both for major elements and for isotopes so it is not possible to identify the majority of samples, nor have any identifiable syenites, trachytes etc been included. A great pity as this is perhaps an extreme example of difference in the isotopic characteristics of oceanic rocks. |
 |
The Austral Cook group.
These include some very low Sr 87/86 of under 0.703, mainly from the basic nephelinites of Tubuai and Mangaia. |
Island Arc and Continental Arc Isotopes
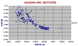 |
Andean Arc Andesites.
This compilation for all studied volcanic centres of the Andes for which we have to thank the efforts of the GEOROC database, shows the basalts and basaltic andesite to have similar isotopic composition to the more enriched members of the ocean basin suite. Points in the upper left are all labelled "rhyolite", "granite" and lower down, "dacite".
We will later try to distinguish "ignimbrite" members. If this series is indeed mainly composed of partial melts of oceanic crust, this is the disposition we might expect. |
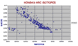 |
Honshu Arc Isotopes.
While the disposition is similar to the Andes note that the basaltic members are more "primitive", ie have low Sr 87/86 and higher Nd 143/144.
The group of low Nd ratio are again the result of a different standardisation factor being used and will be corrected if possible. |
 |
Isotopes for the Aeolian Arc, ( N. of Sicily)
Lower Sr 87/86 are Salina and Vucano, the higher Sr ratio points are Stromboli. (See "Andesites") . Vesuvius in the Roman Province plots along the extrapolation, at Nd 0.51222 and Sr = 0.71
|
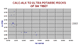 |
Isotopes from andesitic rocks from regions of great crustal thickness.
The four groups are from right,
(1) The Himalayan batholith.
(2) Calc-alkaline andesites
(3) Potassic andesites.
(4) Ultrapotassic andesites.
from Miller,C. et al, 1999, J.Pet. 40,(9), 1399-1424.
|
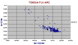 |
Tonga - Fijian Arc isotopes.
The IAB's (Island Arc Basalts) are here more primitive than the Honshu Arc. Erratic points of high Sr 87/86 are illitic clays and sediments drilled from the Tonga-Kermadec trench, which have a fingerprint similar to andesites but enriched in K group elements and in Sr 87/86 |
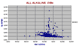 |
Nd vs Sr All Alkaline OIBs. |
Isotopes of Pb in
MORBs, and OIB's
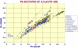 |
Pb 206/204 vs Pb 208/204 for Atlantic Ocean OIB |
 |
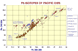
Pb Indian Ocean OIB |
 |
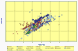
Pb Pacific Ocean OIB |
 |
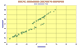
Comparison of Pb isotopes for all arcs.
As presented at Fall AGU, 2002 by B.Sarbas. |
 |
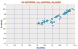
Extreme range of lead isotopes from the Archaean metabasalts of the Baltic Craton. The low group SHOULD be from the komatiitic rocks but most of the sample were not analysed for ME. They show some correlation with silica. |
 |
Os isotopes, no others as yet for comparison. |
 |
Austral Islands (Tubuai) Pb 206/204 vs Pb 208/204 |
The lead isotopes usually
used in geochemistry are:
|
common, non-radiogenic Pb 204 |
|
|
Pb 206, which is the final daughter product of |
U 238 |
|
Pb 207, which is the final daughter product of |
U 235 |
| and |
Pb 208, which is the final daughter product of |
Th 242 |
The radiogenic isotopes are usually ratioed against the stable Pb 204.
A plot of
Pb 206/204
against Pb 207/204 is quite flat as
207 has a shorter half life and as about 4.5 billion years have elapsed to the original setting of the radiogenic clock in the mantle, about 90% of
the U 235 has been used up compared to 50% of U 238.
We have accordingly used mainly ratios of Pb 208/204 but while the ratio of Th/U is fairly constant being
near 3.7 for MORB primary melts, there are some small variations and possibly variations in the parental mantle reservoir so another complexity, even though slight, is introduced.
The ratio of
U 238 to Pb 204 is given the greek constant mu, so "high mu" rocks include the Tubuai nephelinites which have higher Pb 206/204 than other rocks.
Most OIB's lie along a common trend sometimes called the Northern Hemisphere Reference line, (NHRL).
Why Northern Hemisphere? Because some of the southern hemisphere OIB islands are not only more potassic but have a higher 207 and 208 relative to 204, compared to northern hemisphere rocks. The area covered by this
poorly defined southern group is called the Dupal anomaly after the discoverers, Dupre and Allegre, (1983).
As most MORB and OIB have ages not greater than usually 2 -5 million years and maybe only hours, there has been little time for radiogenic breakdown of isotopes with half lives
of a billion years or so. Therefore the isotopic composition should close to that of the source which if uniform, should be constant and all modern rocks should form a single point on an isotope diagram. As the diagrams below shows this is far from the case.
Th and U are more compatible than Pb and so should be and are enriched in any partial melt relative to mantle but the Pb 206/204 etc ratios of young rocks might be thought to remain the same as in the mantle, instead of which we see a range of Pb 206/204 from 17.5 to about 21, the MORB tholeiites being low left and the EMORBS, basanites, phonolites, nephelinites top right.
When we look at the isotopic data of Kamenetsky et al, (2000, and unpublished data) it can be seen beyond any reasonable doubt that enriched low degree EMORB partial mantle melts have higher isotopic values that the high degree LILE-depleted melts. Other very "primitive" high percent NMORB melts of the mantle such as those of Kolbeinsey Ridge, the Theistareykir flows of N. Iceland, and the EPR have even lower isotopic ratios as well as lower Nb/Zr and La/Lu. All enriched EMORBs have high ratios and more alkaline "normal" basanites etc, are almost as high but not as high as the lowest degree, high silica (49-50%) EMORB melts which however are not common. Almost all rock series show a range of linear low-high isotopic variation which does not seem to have been heretofore explained.
We seemingly must first accept that isotopic enrichment does in fact take place in:
- Tholeiitic unfractionated EMORB rocks of a low degree of melt when compared to higher degree melts of
NMORB.
- In
tholeiitic OIB partial melt series, eg, Iceland, Hawaii.
- In seemingly fractionated alkaline basalt series and while the isotope ratio does not change with fractionation, it may with lessening degree of melt even in alkaline rocks. Because of lack of glass only studies we cannot as yet distinguish crystal fractionated from partial melt series in alkaline rocks, except by their isotopes which is to argue in a circle. Relative isotope ratio does not commonly change with alkalinity, some transitional-alkali basalt-basanite series are quite linear isotopically and may overlap longtitudinally, eg those of the northern EARZ.
"Normal" alkali basalt - basanite series though showing quite marked differences in Zr/Nb or La/Sm or La/Lu,
may lie on the same Pb isotopic trend.
- In dacitic lower degree partial melt Arc rocks relative to basaltic andesite higher degree melts of
oceanic crust.
The Mt Washington andesite series of Conrey et al, (2001, C.Min.Pet. 141: 710-732) show a linear array of Pb isotopes that
overlap completely
the Macquarie melts suggesting a derivation mainly by partial melting of NMORB. A few more enriched basaltic andesites may suggest some EMORB was also involved.
More alkaline rocks have both higher
absolute Th-U and higher Th/Pb and U/Pb, whether this is due to the deeper mantle being enriched or due to conditions of melting under higher pressure, cannot be ascertained.
Causes of Isotope enrichment in EMORBs
The most probably explanation for the isotopic enrichment of small degree melts in lavas of low age is that while U-Th are similar incompatible elements, Pb is both more compatible and of different character being divalent or trivalent, while U-Th are quadrivalent. Radiogenic lead formed in a mineral such as monazite, must be in a distinctly "uncomfortable" situation. Pb is also more covalent and can form sulfides, Th and U do not.
In the event of a partial melting taking place, all radiogenic lead, mixed in an intimate atom by atom basis with U and Th in a lattice under considerable strain, is likely to be expelled into the liquid, which will then have higher isotopic lead in proportion to Pb 204 which is likely to be in a different mineral in the mantle material.
The trend of a greater degree of isotopic lead in enriched melts is therefore what we might expect.
Similarly, monovalent Rb 87 is non-mineralogically compatible with divalent Sr 87 and we might expect relative enrichment of radiogenic Sr compared to Sr 86 in a lower degree partial melt. Again, Kamanentsky's Macquarie data show this is in fact the case.
"High mu" samples may be due to derivation from deeper mantle of
lower Th/U ratio. This cannot be proved as disappointingly few studies have included determination of Pb, Th and U. Dupal anomalies may result from sources of higher Th/U and higher K, but again some high K series such as Jan Mayen to not have abnormal Pb 208/204, and there is little or no data for the principle examples, Tristan and Gough.
Conclusions
It does not seem possibly to logically explain the large isotopic differences between the higher fractionates of the sea floor and island tholeiites and that of the sodic and potassic alkaline islands. While higher 87/86 and lower 143/144 ratios are seen in the potassic islands Tristan da Cunha, Heard, and Kergelen, they are not seen in the potassic Jan Mayen but quite similar isotope distribution is seen in the Society Islands which are not at all potassic.
Investigation is handicapped by inability to identify many of the samples used. Hundreds of samples, without major element analysis, are simply labelled "basalt". No consistent correlation with major or trace composition has yet been found, though isotopic consistency is usually found in single centres.
BACK
Copyright © 1998-2003 Dr B.M.Gunn
|