|
GLOBAL DISTRIBUTION OF ELEMENTS Elements covered include:
Preamble As of recent years we have accumulated a great deal of new and more accurate data on the composition of common rocks of the Earth's crust. Many elements have been added to the list which previously were below the detection limit of the methods even ten years ago. Perhaps a fresh look at the world wide distribution of the 100-odd element that God, providence, inanimate evolution or what-would-you, gave us to play with, is overdue. Within the last 5 years, data on trace element levels in common minerals is also beginning to appear, mainly because of the development of laser ablation technology. Global abundances, I should stress, are not in my particular field, there may be up-to-date summaries I am not aware of. But as I have probably looked at more geochemical data than any other person, what is shown below should be factually correct. Discussion of the "whys" of the distributions seen including the degree of partial melt of mantle, differing pressure of partial melt, the nature of the primary or secondary parent rock and fractionation effects are discussed elsewhere. Statistically, a figure for, e.g., "Average K2O in 20,000 basalts of Ocean Ridge Origin" may have some significance though only if we include only glass samples and exclude all possible alkaline rocks, but then as even the tholeiitic NMORB to EMORB may range from say, 0.1 to 3% K2O with decreasing degree of melt and from 1 to 60ppm Rb, what meaning does an average have? For several elements as we have shown in the "MORBs" chapter, the histogram may be quite wide for e.g., Fe, or Ti, though in others quite narrow, e.g. for Si. We have shown, for some elements including Fe+Mg, Ca, Al, Si and Mg, for the bulk of MORB basalts the range is limited, tens of thousands of analysed MORB basalts lie between 49 and 51% SiO2 for example and plot in the "basalt triangle" on an Al-Mg-Ca diagram. Unfortunately MORBs even in this range may include both high-Mg basalt and ferrobasalt, as well as depleted N-MORB and enriched EMORB while alkaline EORB rocks are found on many off-ridge seamounts. EMORBs are found in very slow spreading centres or failed or failing ridges or along the margins of active ridges and seem to be low pressure, very low degree partial melt. The LILE content may be greatly elevated, eg Nb>100ppm, La ~50ppm but the fractionation trends remain mainly tholeiitic (i.e. ol+cpx+plag) though the plagioclase appears later in the EMORBs. They can be distinguished from alkaline series by the steep rise in K relative to soda, the very flat La/Lu and Nb/Y and also by the relation of Fe to K, the cumulative ankaramites often having fairly constant 14% iron but no ferro-basalt peak. One of the tasks at this point is to be able to locate rocks with abnormally high and low concentrations of elements for several reasons. One is that these may pose a health risk, eg neither humans nor animals nor in some cases plants can live on land or soils derived from rocks deficient in Bo, Co, Zn, Li, Cu, Fe, K, P, Ca, to name a few essential elements, while high levels of Se, As, Cd, Pb, Hg, U, Th, especially can also be a danger. For most elements we can only tolerate a range of about +/- a factor of 4 on either side of the mean crustal composition, a factor that people who see us voyaging to new planets within a few centuries might do well to ponder. Metals are essential to modern civilisation and we expend enormous amounts locating high concentrations and ores of most of the elements in the periodic table. When we realise we consume 72,000 tons of yellow cake uranium oxide per year and that western countries use 1/4 ton of steel per head of population per year and one sees 290,000 tons of 60% iron ore being hauled out of Mt Tom Price and Mt Newman per day, it gets quite frightening. By 2007 annual steel production will exceed a billion tons! What rocks are likely to contain the highest Sc? Nb? Ta? All are essential in the 21st century. Rock divisions used As said elsewhere there are two major divisions of crustal rocks, those
derived from sub-oceanic mantle, and those derived from sub-continental
mantle. These two have fundamentally different fingerprints and different
fractionation patterns and are very difficult to compare. In the oceanic basins we see two main types of rock, the Oceanic Ridge Basalts, stemming from the mid-ocean spreading ridges, and the more alkaline Oceanic Island Basalts seen in scattered localised island groups. On the continents there are also two main rock suites, the Orogenic Andesites forming chains of composite cones along continental margins and along incipient continental margins on offshore arcs; then we have the Continental Flood Baslts as seen at Columbia River, or in the Siberian Traps, with the same continental signature, but with their low alumina and high Fe,Ti, quite distinct from the andesite series. Tholeiitic (or Qz-saturated) rocks are dominated by olivine and ol+cpx+plag fractionation followed by TiMt and by apatite, with a residuum of a quartz-sanidine graphic intergrowth. Large changes in Fe/Mg ratio can occur with fractionation of olivine and pyroxene and a large build-up in residual elements may take place at almost constant silica content until the TiMt point is reach when silica begins to build up, Ti-magnetite containing no silica. In large areas of the oceanic environment rocks of over even 52% silica may be entirely absent though in rare cases a dacitic rock of 65-68 % silica may be found along with intermediates. However it must be realised that the name "tholeiite" has been applied to Flood Basalts, to OIBs, to Ridge Basalts and also to the more depleted island arc members of the calc-alkaline or andesite series. There is genetically no such rock as a a "tholeiite", each one of the four main rock series may include quartz saturated members.. On some seamounts and most oceanic islands, alkali basalt begins to crystallise along an olivine-clinopyroxene cotectic, which may be 50-50 Ol-Cpx or 40-60 or perhaps lower. Many trace elements are differentially taken up by clinopyroxene and this difference in the crystal cumulate can have profound effects. Sialic rocks fractionate to a more limited extent by removal of horneblende or a more iron-rich cpx + Opx and a more sodic plagioclase. The cumulate members correspond to the eucrite blocks often found, but fractionation is seldom strong in sialic rocks. At least some of the compositional shift from basaltic andesite-dacite is the result of different degrees of partial melt in the sub-continental lithosphere. There is a steady build-up of the K-group elements with compositional increase in silica, and in whole regions there may be no rocks of less than 54% SiO2 and often none with more than 64%. . So we cannot compare the two groups by comparing abundances of different elements at a fixed silica percentage because of the small range of silica in MORB series and lack of overlap except in a handful of cases. Likewise the range in Mg, (Fe+Mg)/Mg or Mg# in sialic rocks is quite small (usually 4% to 0% MgO) while the silica range may be large, (52 - 76%), but the rhyolitic members often appear to be third stage remelts of continental crust. The Recovery of Metals from Low Grade Ores We are indebted for the following account of modern metal extraction methods to Mr Mike Banks, an economic geologist and consultant operating mainly in the SE Asia, Australasian area and based in Christchurch, NZ. E-mail: mjbanks@clear.net.nz. Gold Extraction With the exception of alluvial gold (still concentrated primarily by gravity methods), the majority of world production of the metal is now extracted from bulk hard-rock ore by variations of the cyanide method. Gold and silver are capable of dissolving in aqueous alkali cyanide solutions, where following concentration and clarification the commodities can be recovered by precipitation onto a metal substrate collector. Introduction of the cyanide process (first in New Zealand in 1889) revolutionized gold extraction efficiency, resulting in much low-grade ore becoming economical to mine. In the 1980's heap-leaching of ore stockpiles by cyanide solutions, without the need for fine-grinding, allowed very low grade (1.5g Au/tonne or lower) ore to become economic to process. Nowadays, where the gold in the rock is in the form of free metallic grains (free-milling ore), but so dispersed that fine milling still being necessary, the cyanide solution is added to the finely ground rock, along with activated carbon granules (which directly adsorb the gold from the cyanide solution in-situ. This process termed carbon-in-pulp (CIP) was developed in South Africa (circa 1975) and has resulted in greater extraction & cost efficiencies. Where the gold is not free-milling (refractory ore -about one third of the world's production, enclosed in sulphide or sulphosalt minerals), ore may have to be roasted, pressure oxidised, or bacteriologically oxidised to free it prior to dissolution by cyanide. Modern Copper, Lead and Zinc Extraction In the past, recovery of these metals generally required fine-grinding of sulphide ores, froth flotation, roasting, smelting and electrolytic refining. These processes (while still necessary for many ores) are expensive, time consuming and environmentally undesirable. Increasingly however, leaching of large volumes of broken cobbles of oxidised base-metal ore, has proven far superior in terms of simplicity, efficiency, cost and environmental gains. In the case of copper, over 20% of this metal is now derived from the solvent extraction, electro-winning (SX/EW) process. Sulphide ores too, can be subjected to bacterial oxidation prior to SX/EW or incorporated into the process, where mixed oxidised/ sulphide ores are present. Basically, SX/EW involves adding weak sulphuric acid (raffinate) to broken ore either in a pre-mixer, or on the leach pads. Additions of ferrous salts initiates bacterially assisted oxidation of sulphide minerals, whilst the acid leaches metal oxides. Raffinate is applied to the top surface or ore pads and along with atmospheric oxygen trickles by gravity through the ore to be collected and pumped to the solvent extraction plant. In the SX plant, the aqueous pregnant leach solution (PLS, raffinate loaded with copper) is circulated in intimate contact with an organic solvent (kerosine) containing a copper selective reagent (extractant). The two immiscible liquids are separated by settling after passage through each extraction cell, the depleted raffinate piped back to the leach pads, the organic extractant solution to the next cell for mixing with more PLS. After passage through multiple cells, the organic extractant liquid is fully charged with copper and piped to the stripping stage where it's copper content is stripped by contact with a concentrated aqueous solution of sulphuric acid. The stripped organic liquid goes back to the first stage cell in the extraction circuit. The concentrated acid copper solution (electrolyte) is filtered to remove particulate and organic contaminants and piped to the EW (electro-winning) plant. In the EW plant, the copper in the electrolyte is precipitated onto stainless steel cathode sheets, by a low voltage DC current passed through the electrolyte. Sheets of high purity (99.99%) copper metal are mechanically removed from the cathodes, stacked on pallets and transported to the market. Modern Models for the Genesis of Copper, Lead, Zinc Deposits The bulk of the world's copper, lead and zinc is currently produced from four classes of occurrence: Redact (Redox) Sedimentary deposits, Volcanogenic Massive Sulphide (VMS), Sedimentary Exhalative (SEDEX) deposits and Porphyry deposits. Although the geological and tectonic settings of these deposits are extremely diverse, their geneses all seem to be the transport of the metal by aqueous halide solutions (predominantly chlorides), and reaction of these brines with associated sulphur complexes (or oxygen) to precipitate sulphides (or oxides). Concentrated base-metal transporting brines are generated by exsolution from felsic magma; (Porphyry copper/skarns), superheated ocean water/magmatic exsolution; (VMS), hydrothermal ocean brines; (SEDEX) and deep basin/rift generated pore-water brines; (Redox deposits). In the past two decades, precipitation of base metal suphides by the rapid cooling of hot brines, has been directly observed from submersibles in the world's oceans. These observations of "black (and white) smokers" and analyses of the deposits from them, leave little doubt that VMS and SEDEX deposits have resulted from the prolonged action of these plumes of super-heated, metal chloride-rich fluids in the past. This general group of giant base-metal deposits also produce a host of associated metals, notably molybdenum, gold, rhenium, silver, indium, cobalt, vanadium, uranium and others. By: Mike Banks Aluminium Al makes up about 8% of the Earth's crust being the third most common element after oxygen and silicon. As the oxide Al2O3, it is found in feldspathic and clay minerals in virtually all silicate rocks and soils in the range of 12-20%. In spite of this, it was not produced in quantity as a metal until 1886 being an oxide very difficult to reduce. The big aluminium smelters Alcan, Alcoa, Pechiney, Billiton, Comalco still electrolyse with graphite electrodes using huge amounts of power - about 6.8 KwHr/lb or 63000 BTU's per lb. Reduction of the hydroxide to the oxide is done under pressure in autoclaves with in tall seeded towers at temperatures of, as I recall, less than the usual 220ºC. The big ALCAN plant at Arvida (Quebec) was once almost shut down because of the presence of boehmite which is stable at the operating temperatures used and was seeding all the alumina out as boehmite, not as Al2O3. They could only use an ore very low in this mineral at Arvida. We were able to devise a method of analysis using a graphite monochromator on an XRD machine that could detect down to about 0.1% and the boehmite-bearing ores could be sent elsewhere. We were very popular with ALCAN for a time! Al Consumption and Uses In spite of the complexities of production, billet Al metal is currently priced at approx $1/lb, world production being about 22,000,000 tons. Again this is difficult to allocate to country as the big smelters are international, the Bluff Smelter in New Zealand which uses most of the power produced at Lake Manapouri in Fiordland, is owned by an Alcan-Alcoa consortium. Al2O3 in Silicate Rocks Al2O3 is usually found near 14% levels in basalts and due to it's presence first in plagioclase then in potash feldspar, and feldspathoids, it changes little with fractionation. It may decline slightly in tholeiitic rocks to 12% and increases in alkaline rocks, especially trachytes and phonolites to 17 - 20%. It also reaches 17-18% levels in andesites but declines to 14% in rhyolites-granites. I have found 45% Al2O3 in laterites, but what is found on commercial open-cast mines I do not know at this point but pure Gibbsite (Al(OH)3) has about 65%. Common aluminous clays containing silica can be used as Al ores but not economically at this time. Silver, Ag Ag (At.No. 47), while too rare to be of interest petrologically is still of large economic significance. It is ironic that in the time of Croesus, credited with being the first to mint coins in about 760 BC, they could control adulteration of the coinage by means of the colour of the streak of precious metal on a piece of slate or touchstone. Gold is pale yellow, with added copper it becomes red, with silver much paler. The scarcity of precious metal restricted inflation, the Athenians were only able to fight the Persians at sea because of the discovery of new ores at Mt Aurum which produced at it's height about 1 million oz a year. It has been claimed that the biggest theft of all time took place when the US Govt. recalled all gold and silver dollars and replaced them with worthless paper. Only 30 years ago the US and Canadian silver quarters were recalled and replaced by cupro-nickel of virtually no intrinsic value. History is a fascinating subject! Different mining and materials resource organisations give highly variable figures for total annual silver mined, ranging from 12 - 18,000 tonnes. MBendi Mining News gives 16,470 tonnes.
Fortunately Ag is recovered as a by-product of Pb operations (remember the 10oz Ag per ton of galena at Broken Hill?) or Cu-Zn as on it's own, most Ag recovery could not be economic. While silver plate and table ware is no longer popular, Ag finds heavy use in the photographic industry because of it's sensitivity to ionisation by light. This use is declining rapidly with the edvent of the digicam, the "film" for which is much cheaper. In many countries, eg India, Mexico, Indonesia, silver, heavily alloyed with Cu, is extensively used for jewelry. Gold Gold has been mined for at least 5000 years, and the granitic terranes of Saudi Arabia are pock marked by ancient "King Solomon's Mines". Silver was even more important and tributes or taxes were assessed and paid to the "Great King" of Persia in classical times in talents of silver. Herodotus lists the taxes of the main eastern Mediterranean countries and quite small provinces such as Cilicia and Cappadocia were assessed at several hundred talents, a Euboean talent weighing 64lb. Production in New Zealand In the Coromandel fields about 51 million ounces of silver and 10.5 million ounces of gold have been taken. The Martha Mine is still operating, the solution techniques for dissolving gold from finely milled or being very efficient. A million oz. is approx 37.2 tons. (at 12 oz Troy/lb)
World production In 1847 the total world production was 75t of which half came from Russia. Then came the series of classical gold rushes to rich placer deposits.
These figures are according to the World Gold Council. Barium Ba++ occurs in silicates mainly in potash feldspars substituting for K. The main source is as BaSO4 (barytes) found in evaporites in desert salinas. About 5% of the very large industrial use is as a filler in plastics. 95% is used by the drilling, (mainly oil) industry as a drilling mud because of it's high specific gravity. It both lubricates the drill string and prevents the uncased hole from collapsing. Ba in MORBs and OIBs Ba varies widely with increasing alkalinity, tholeiitic basalts such as Puu Oo in Hawaii having 100-120ppm, K/Ba=33, compared to 300-500 in alkali basalts-basanites in which the K/Ba =30 for Tristan, 26 for Vesteris seamount, ~35 for St Helena, 32 for Mt Sidely, 23 for the Tubuai nephelinites etc.
Ba in CFBs Ba is always elevated in CFB rocks along with the Rb, Cs. Th, U and
may reach very high levels. Ordinary CFB basakts may be int he range
200-300ppm in but in fractionated small degree melts, Ba goes out the
roof. This in the Umatilla Basalts of the Columbia River, Ba reaches
4000 ppm and in the alkaline late stage Snake River 3200 ppm. Ba in Sialic Rocks One can only say Ba is extremely variable. In the south-central Chilean group, the centres for which there is reasonable data follow what we will call the Puyehue trend. These include volcanoes Planchon Petaroa, Azufre, Llaima, Villarica, Copahue, Co. Antuco etc with a Ba(60) ie, a Ba content at 60% silica, of 500. Laguna del Maule at 36S also has a Ba(60) of 500 but when plotted against silica has a less steep trend. (Frey et al, 1984). Ba Anomalies and Ba/Rb Many orogenic series show variable Ba seen in fingerprint diagrams as a positive or negative anomaly relative to Rb and Th. Volcanic centres showing a -ve anomaly (given as highest level/EMORb ratio) include Sakurajima, (5); Puyehue, (10); Lascar, (7); Cerro Tuzgle, (4); Taal, (Luzon), (7); Negros Id, (8); Vesuvius, (7) and Santorini, (5.5) Distribution of Barium in OIBs
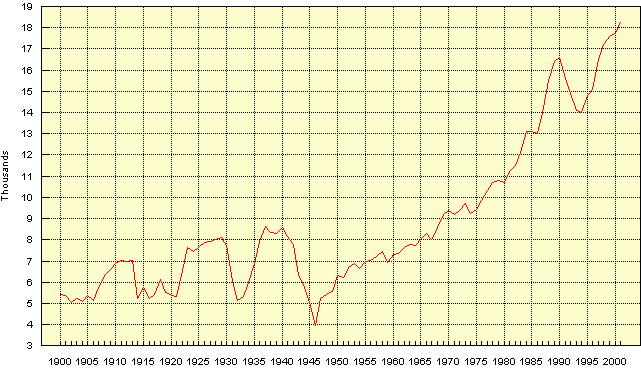
Carbon (coal and oil) These have nothing to do with igneous geochemistry but while we are looking at the frightening metal consumption figures, let us take a look at those for coal and oil. These resources are mainly used in energy production, though for coal up to 25% is used in some countries as a reducing agent in iron & steel production and 10% in cement production.
(The barrel being the standard 44 gal imperial = 50gal US drum = 159 litres) These numbers arouse similar feelings to those expressed by the Iron Duke as he reviewed some of his less impressive troops before the Peninsula campaign, "They may not frighten the French, but by --- they frighten me!"
Diamonds, like gold do not have any great intrinsic value except as an abrasive, diamond being one of the very hardest materials known. Relative to weight they are the most highly priced of known minerals, animals or vegetables, and all because they sparkle in a light beam. So does zircon and most people would not know the difference. However... Consumption According to the World Diamond Council, the world production for 1999 was:
The world total being 111,038,000 carats, or 22.2 tons, which is quite a heap. However, if of 6 billion people, 3 billion are female, and on average 1 in 50 are married each year and is given a 1 carat diamond ring, half the production must be gem quality. In the Argyle mine (actually situated on "Stumpy Michael's" old Lissadell Station, not on Patsy Durack's Argyle which is now underwater, see "Grass Castles in Air" by Mary Durack), only about 5% of stones recovered are gem quality, 55% are industrial. The latest diamond field find is that of two Kimberlite pipes in the Otish Mountains of northern Quebec by Ashton Canada and associates. (Graphite) Graphite is a polymorph of carbon and diamond, being formed of hexagonal sheets of C. These slide over one and other giving it its good lubrication qualities. Uses of Graphite Graphite is of course used in "lead" pencils, and as the anode in C-Zn alkaline battery cells. Latterly there is prophesied to be a great expansion in graphite use in fuel cells. It is used as electrodes in both electric Bessamer furnaces used in steel production and in the electrolytic reduction of alumina, the electrodes I have seen being 6 - 10in diam. Graphite stands considerable heat, especially if oxygen is excluded. We used graphite crucibles for many years for melting rock-powder + lithium tetraborate for XRF analysis at about 750 deg C. The graphite slowly burned away but the crucibles could be used 2-3 times and were cheaper that Pt-Au. It is used as an electrically conductive lubricant and as a self-lubricating seal in some water pumps and oil pumps. Sources and Production of Graphite Graphite needs moderately high pressure to form and occurs in graphitic marbles that have been subject to amphibolite to granulite facies metamorphism. Up to 15% of the rock may consist of rounded lumps of graphite. Weathered marble for preference is crushed and the graphite separated. Graphitic shales also occur, but are not usually used as "ore". Carbon dioxide Of course, all these horrendous amounts of oil and coal and wood and natural methane-propane-butane gas (84.2 trillion cub. ft. for 1999) consumed by the six billion people that we share this now relatively small planet with, end up to a large extent as CO2 and is emitted into that vast sink - the atmosphere. Wood is the major source of heat and cooking fuel in undeveloped countries so perhaps this should be discussed along with the usage of coal, oil and gas. Wood consumption has declined slightly as more countries have cleared their forest so that huge populations no longer have access to it, and its place is taken by coal, oil, gas, with electricity from hydro -power and from atomic energy. Between about 1960 and 1988 wood consumption was fairly static at 0.65 cubic m per person per year, but since 1988 it has declined to 0.55 m3/cap. The forested areas have halved in about the last half century to about 3 billion ha, and continues to decline as planting has, on average been minimal. The International trade in wood at $130 billion per year is the third largest commodity, but world wide 63% of all wood is burned as fuel with industrial wood use accounting for 1.05 billion m3 according to Dr South of Auburn University, USA. The US, with 5% of the world’s population consumes 20% of the world's wood. The carbon dioxide produced from man-made industrial emissions averages about a metric ton of C per person, though in some small Arabian oil-producing states it can be as high as 25t per capita because of flaring off of gas at oil refineries. With the setting fire to the Kuwaiti oil wells by the Iraqis when they were expelled from the country, 130Mt of C were released in 1991 alone. An industrial state like Germany releases 216 million tons, (or 2.63 t per capita), more than the total for the whole of Latin America. World wide emissions in millions of tons C for the industrial countries in 1999 were 3649Mt and a further 2621Mt for the "developing" countries. Above figures come from Marland, Bodin and Andres, "2002 Global, Regional and National CO2 Emissions". Cadmium Cd (= El. No. 48) has similar properties to Zn and is found in zinc ores as CdS substituting for ZnS. It is occasionally found pure as Greenockite. It is recovered during the electrowinning process at a rate of approx. 3 kgm/ton Zn. Prices are erratic having recently dropped from about $12/lb five years ago to approx. $1. Worldwide productivity figures have not been found but US productivity recently was 1100t. Cd finds uses in NiCad batteries, in plating fasteners, and in other electroplating. It has a low temperature of evaporation of 767 deg C which probably explains its use as a cheap rust inhibitor in place of Zn. However a cadmium coating wears off quickly and is not nearly so effective as Zn, however it keeps cheap fasteners looking presentable in a shop. It also finds application as a neutron absorber in nuclear research. It is poisonous as are other heavy metals Pb and Hg, and one wonders about welders who casually weld Cd clad steel. As Zn is only present at about 80 ppm levels in basalts, it can be guessed that Cd is present at sub ppm levels and is not of great interest to the petrologist. However, Kamenetsky's data for the Macquarie parental melts show a linear range in Cd from 0.15 in NMORB to 4.25 ppm in extreme EMORBs. That is no non-accumulative basalt can have less than 0.15ppm!! Ca/Na As these two elements are so intimately associated (as are Mg, Ni, Fe; or Zr,Hf) they can be considered together. In primary rocks these two elements are present mainly in the plagioclase feldspar solid solution series with the two end-members anorthite (CaAl2Si2O8) and albite (NaAlSi3O8). Anorthite is the high temperature end member being divalent so that the early crystals forming in a basalt will be calcium-rich of perhaps An 70-80, ( i.e., 70-80% of the Ca end member) while dacites or rhyolites might contain oligoclase or albite of An 30-10. Feldspars tend to weather mainly to clays. The Na content of Ca-rich pyroxenes is surprisingly low, so that pyroxene formation may alter the Ca/Na ratio rapidly. NaCl use We currently use about 214 mt of NaCl mainly domestically, of which about 1/3 is obtained by solar evaporation of sea-water, about a third by mining sold rock salt (halite) and a third from brines. Australia produces about 8.5mt from solar evaporation, worth some $300 million, as seen near the Karatha, Roebuck Bay area. NaOH, the ultimate alkali, used in so many industrial applications is now produced by the electrolysis of NaCl. Sodium metal while a good conductor, is soft and far too reactive to be of much application except under carefully controlled conditions. As fresh water becomes a scarce commodity and deserts expand it is a consolation to note that prices as low as $1/m3 are being quoted for desalinated water produced by reverse osmosis. However, calculate what it would cost to fill Lake Superior with desalinated water, before we cheer too loudly. CaCO3 Calcium carbonate or limestone is so widely used for soil pH control, as a fertiliser and as a chemical reagent and is so widespread as sedimentary limestone and marble and is so available that it seem no record is kept of it's bulk use which probably hits the billion tons mark. Some carbonate occurs in primary carbonatite magmas but by far the greatest amount forms from the breakdown of anorthite minerals during weathering and reaction with atmospheric CO2. Ca is in solution in the sea as the bicarbonate. Excess atmospheric CO2 is absorbed to form insoluble CaCO3, however there is still a definite (short-term?) increase in atmospheric CO2. Anorthite-Albite The crystallisation of all basaltic magma results in more Ca and less Na being removed in the first formed crystals than is in the magma. In actual rocks at least half the decalcification is brought about by the simultaneous formation of clinopyroxene which contains about 20% CaO and negligible soda. After protracted fractionation most of the Ca will have been removed and a great deal of Na will remain. Phonolites which are the end product of fractionation of basanite may have up to 10% Na2O and less than 1% CaO, the basanite having about 10% CaO and perhaps 2.5% Na2O. A few other elements are affected by this process, eg alumina will increase in phonolites to about 21% or more from a starting point of about 14%, Sr will build up and then decline with the incoming of sanidine and sodic feldspar. Na and Ca in primary MORBs & OIBs The Macquarie primary melts show a decline in CaO and increase in Na2O from large to small scale melts from 2.3 to 4.2% Na2O. This explains why we see the same range in different MORBs. K increases with Na but more steeply. Na and Ca in Arc Andesites The more primitive arcs, (Scotia Arc, IBM, Tonga) are quite calcic with basalts at 13% CaO, 1.5% Na2O trending towards soda dacites at <1% CaO and 7.5% Na2O. The presence of the high soda Deception Island, discussed elsewhere, lead to two separate trends for the Scotia Arc. Cobalt Co has a lower partition coefficient than Ni in olivine. In the 1959 Kilauea picrites I found 250 ppm Co in olivine compared to 60 in the undifferentiated melt, so we have a partition coefficient xtl/liq of about 4 compared to 13 for Ni. Co production Cobalt is used in special corrosion resistant and High Temp. alloys,
also as a pigment in ceramics ("cobalt blue"). Co in MORBs and OIBs The most basic glasses from Kolbeinsey Ridge north of Iceland , have 50 ppm, from SEIR 40-50 ppm and from the EPR 40-50ppm. Undifferentiated Kilauea has about 60 and Loihi 50.
Co in Sialic Orogenic Andesites All andesitic rocks seem to show a similar trend of a shallow dome shaped distribution with a maximum at about 30-40 ppm and 5% MgO. No significant difference is seen between island arcs and Mt Vesuvius. Chromium The behaviour of Cr is at first sight very like that of Ni, it is very high in picrites and dunites or serpentinites and low everywhere else. However trivalent Cr+++ does not enter into olivine but forms a Cr spinel, the very first mineral to form in a basalt. Tiny grains of spinel get grown into olivine and when the olivine (or orthopyroxene) accumulates, so does the Cr. In extreme fractionated basaltic intrusions of great size such as the Bushveldt or the Stillwater, chromite may form it's own cumulate layers, almost pure. Mining chrome is in such cases very simple which may explain its current very low price of $85/ton. Cr uses and consumption The main uses of chrome being in plating, and in stainless steels and alloys it is surprising that the world production is now 12 million tons of chromite, which makes one wonder what would happen if chromed bumper bars came back into fashion. Two thirds of this come from Australia and the ex-USSR. Chrome in MORBs Kolbeinsey Ridge glasses have a maximum of 400 ppm as has site 504 in the Galapagos Rise. The EPR has slightly less at 300 ppm (Regelous, 1999); 320 ppm (Niu & Batiza, 1997); and 340 ppm (Niu et al, (1999). Cumulative harzburgites from the Garret Transform have 3000ppm (Niu & Hekinian) Parental melts in ORBs have 120 - 400 ppm in rocks of 6 - 10.2% MgO so that any ORB basaltic glass with more than 400 ppm should be treated with suspicion.. Cr in OIB picrites and peridotites Mauna Loa, Mauna Kea, Kilauea Iki, and Piton de la Fournaise picrites
all have a maximum Cr of about 1900 ppm in picrites of 22.5% MgO which
extrapolates to a pure olivine with about 3500ppm. The trends are less
regular than seen for Ni and the different centres are not distinguishable. Cr in Alkali Basalts The OIBs St Helena, Tristan, Gough, Cape Verde Is. etc again have slightly variable trends on different islands with a maximum in cumulates of about 1000ppm.
Cr in Orogenic Andesites. The great bulk of orogenic andesites only rarely exceed 7% MgO and 150-160ppm Cr. Caesium Cs is atomic No. 55 and has no less than 32 isotopes. The single outer orbital electron makes it extremely ionic and is the most electropositive and alkaline of all metals. Pure Cs, is, like Ga and Hg, liquid at room temperatures. It occurs mainly in the minerals pollucite and lepidolite but a single pollucite deposit at Bernie Lake, Man, is claimed to have 300,000 tons of pollucite with 20% Cs. At Lac du Bonet this is being developed into a heavy drilling mud which seems to be a scandalous waste of a rare element. Pollucite forms a dicontinuous series with analcite and has a composition CsSi2AlO6. Cs is used in radiation detectors and in Cs clocks, the latest Cs clock at the National Institute of Standards being accurate to <1 sec in 20 million years. It's great attraction for O2 is used to decontaminate electronic tubes. The current price is about $30 per gm. Cs is the most "lithophile" of all elements and shows the greatest relative increase with fractionation, with decreasing degree of melt and with alkalinity. That is, in partial melts, the amount of Cs goes up very steeply, but the ratio of Rb/Cs = 95; Ba/Cs=940 and K/Cs=23,500 remain constant. In MORBs the amount is very lw, often<1ppm and Cs is very prone to alteration so its geochemical behaviour is not so easy to define. Moreover, due to its low concentration, it is only recent data for which Cs is available in any amount though ID determinations for Cs have been made for at least 30 years, they are in no great number. If we compare the EPR MORBs with a tholeiitic island
such as Cerro Azul in the Galapagos we might expect the Cs to be much
higher in the island rocks as are the K, Rb, Ba. We might also expect
that the ratio of K/Cs or Rb/Cs would be somewhat lower. This does not
seem to be the case. Comparing these two group together with the potassic
OIB Heard Id, we find the ratio K/Cs is similar at ~34,000. If we compare
Cerro Azul with the EPR we find Cerro Azul has less Cs relative to Nb,
as Nb goes up very steeply in islands, but it has double the Cs relative
to Zr or to MgO! As there is no or poor data for most OIBs the variation between islands cannot be discussed as yet with any degree of certainty, though good data has appeared in the post 2000 era.. In arc rocks Cs is extremely variable as are all the rest of the potassium group elements. At 2% K2O in the Indonesian rocks the Cs ranges from 2 - 12 ppm while in the Scotia Arc at 0.6% K2O the range is from 0.1 to 0.9 ppm. Cs is high in the ultra-potassic Mt Vesuvius, as might be expected, with a range of 12-22ppm and a K/Cs of 3390, but the Alban Hills, though highly variable have as much as 60ppm (Peccarillo etal, 1984). Cs is very low in amount and highly variable also in Continental Flood Basalts. The CRBs, Parana Basalts and Ferrar Dolerites have a K/Cs in the range 13 - 25,000, that is, they have more Cs than MORB but less than OIB. Copper Cu is one of the most metallic of metals. It is highly ductile, malleable (can be drawn into wire at quite low temperatures) and is one of the best conductors of electricity, narrowly beaten by silver and gold. It is also sonorous (brass bands) and relatively corrosion resistant. However bronze winches at sea tarnish like the very devil, polish them to a mirror and they will be tarnished by a passing rain shower, (but not corroded). Cu was (we believe) the very first metal to be smelted. A hot fire may well have reduced malachite to native metal accidentally. We do know that copper has been used for tools (even hair combs) for close to 10,000 years. Accidental alloying with coexisting tin shortly afterwards gave rise to bronze which is much harder. Today, bronzes and brasses (Cu + Zn) are used in a huge range of alloys. Al may be used for high-transmission lines because of lower cost and weight, but in electric motors and integrated circuits where large savings can be made because of the lower volts required to push electrons through wound armatures, Cu is always preferred. We currently use over 10,000,000 tons Cu per annum, about a third of which is recovered from scrap.
The main ores are of pyrrhotite (CuFeS2), bornite (Cu5FeS4) covellite (CuS) and chalcocite (Cu2S). In the oxidised zone above ore bodies where the primary ore is exposed to atmosphere, a cap of green malachite and or blue azurite may form, these being carbonates-hydroxides. At the famous Bisbee, Arizona mine (now closed) the ore, in addition to malachite-azurite (of which I have a nice sample,) includes a thick vein of pure native copper. The Lavender Pit at Bisbee ended at a 1 mile x 0.5 miles area and 900ft deep, with 46M tons of overburden being removed before the pit began. The Sonoran Desert of Arizona still produces 60% of the total Cu output of the USA. Chile is the world's greatest produce of copper, and the Chuquicamata mine is the biggest. However, concern for the environment is not one of their greatest concerns and dense clouds of sulfur dioxide and arsenic fill the valley, and lung problems among workers and birth defects are common. The United States is next in production followed by Australia and Canada, both producing about 16%. According to the London Metal Market, bar copper was a few years ago selling at $1.20/lb in bulk. No year 2002 data is available as yet. Again, regarding the complexity in mining, crushing, solvent extraction, electro winning, concentration, smelting and final electrolytical purification - such a low price is almost unbelieveable. Very efficient, those fellows. Deposition of Cu Cu sulfides are more volatile at lower temperatures and Cu is more "chalcophile" than most other metals and ores are found in a wide variety of environments. At Sudbury copper ores, mainly chalcopyrite, occur along with nickel in roughly equal amount. In nearby Kidd Creek copper is found in permeable rhyolite tuffs. As rhyolite seldom has more than 10ppm Cu we can assume the ore was introduced. Large gabbroic and komatiitic intrusions below provide a heat source. Old volcanoes in Chile associated with fumaroles which have been emitting sulfur gasses for centuries show copper mineralisation where deeply eroded. Copper ores are associated with either gabbroic rocks or with calc-alkaline volcanics, as in Chile, Peru and the large and controversial Bougainville Copper mine in the Solomon Islands. Cu in MORB rocks Cu in general is found at a maximum of 80 - 300 ppm in basaltic rocks declining linearly to only 5 - 15 in rhyolite - trachyte. Cu also declines with dilution by accumulative olivine, Ol + Cpx, Ol + plag etc so the most Cu-rich magmas are those of 7.5 - 10 % MgO. Cu in OIBs Once again, very few investigations of oceanic islands include Cu. As only the second most important economic metal we have, it does not warrant much attention apparently if one can be permitted a little sarcasm. Cu in Back Arc, Island Arc, Continental andesites etc. Niua Fo'ou west of Samoa has only 85ppm, Site 834 in the Lau basin 70 at 9% MgO while Pearce et al (1995) found 150 ppm also in the Lau basin. The Scotia Arc shows a great deal of scatter with a maximum of 180, the Tonga-Kermadec Arc being similar with up to 200 ppm at 5% MgO. Cu in Continental flood basalt As might be expected in rocks that have parental magmas ranging from <50% to 56% SiO2, the Cu values vary widely being greatest in the more basic members. Thus the more basic Picture Gorge members of the Columbia River basalts have a range of 125 - 350 ppm (P.Hooper, 2002) and the similar Steens Mtn rocks 150-350ppm (Gunn & Watkins, 1971). However the more felsic Umatilla have only an average of 90 ppm. Iron and Steel Fe, (At.No.26, Mol.Wt. 55.845) is not only the mainstay of civilisation and our most important metal, but is one of the more important elements in our understanding of geochemical evolution. Iron is only rarely found native as at Disko Id, Greenland, usually it occurs as hematite (Fe2O3) or as goethite (HFeO2) or limonite (Fe2(OH)3) or siderite (FeCO3). Pyrite (FeS) is mined more for the sulfur content rather than for iron. Current world consumption of steel for 2001 was 840 mt. It is forecast that by 2007 world consumption will exceed 1 billion tons. Current cost of steel coil is $400 US/t. About 25% of steel is recycled. As about 70% of cars in USA are imported it is claimed that the local industry operates on 80% recycled steel, however the energy required per ton of steel remains about the same. Alloyed with C, Ni, Cr, P, Cu, Mn, Mo, Al, etc, steel takes on an incredible range of properties of flexibility, toughness, stress resistance, shock resistance, corrosion resistance, but remains a fairly heavy construction medium hence the increasing use of Al, Al-Mg and Ti metals. Historically iron began to be used in tools or for weapons only in the era 1500BC, the high temperature (900 - 1000º C) needed to reduce the oxide to metal requiring a blast furnace, or at least abundant charcoal and an efficient set of bellows. Regrettably most historians are not engineers or technicians and the historical record of iron mining in Europe is lamentably deficient in information on the ores used. Early Norse iron working was centered around bog iron ore, nodules of limonite found in poorly drained, highly reducing bogs. The historic Norse settlement at l'Anse au Meadows in northern Newfoundland is reported to have samples of worked bog iron. Currently the vast deposits of Archaean sedimentary banded iron ore formation as seen in West Africa or at Mt Newman and Mt Tom Price in NW Australia are the greatest producers. At the latter the bulk ore runs at about 40% iron but secondary enrichment by goethite brings this up to better than 60%, the bulk of the ore being specularite, or adamantine hematite (called locally "microplaty hematite"). Weathering of Archaean greenstones in an atmosphere high in CO2 must have caused leaching by acid rain with a ph of 5 or less. (The transition FeCl3 - Fe(OH3) takes place near pH 4.5). On entering a sea of neutral pH, the iron would be precipitated as the hydroxide and under compression and metamorphism, revert to the oxide. This is an interesting topic and one we are seeking more information on. Ore horizons inches to feet in thickness in the Hammersley Range alternate with aluminous shale and chert. Al(OH)3 precipitates at a pH of about 5.2 and it is surprising at least to me, that pure aluminous horizons are not found. As the CO2 became absorbed into limestones and free oxygen appeared in the atmosphere, this process ceased and there are no known sedimentary iron ores formed after about 1 byr bp. Fe - Mg replacement in Basalts Mg and Fe have much the same ionic radius and charge along with other minor elements such as Mn, Ni, Zn, Co. They can therefore share the same sites in the crystal lattices of pyroxene such as diopside, augiite, hedenbergite, pigeonite, orthopyroxene and olivine. In all cases the order of preference is Ni > Co > Mg > Fe > Mn > Zn. Only Mg and Fe are present in large amount, always > ~3% in basaltic rocks. The first of any mineral series to form will have a high Mg, low Fe content so that the mineral may have, in the middle range, 30% more Mg and 30% less of Fe than the magma it is forming in. This results in rapid depletion of the magma of Mg (and even more of Ni) and build up in the amount of iron until the residual magma may become a ferro-basalt with 18% or more FeO. Not until the MgO reaches 3-4% and titanomagnetite precipitates causing a sudden drop in the amount of Fe, Ti and V does the composition of the crystal residue attain a lower silica content than the magma, and the silica content begins to rise. There is not a great deal to say about Fe in igneous rocks. The range can be 3 -18% in MORBs and about the same in Continental Flood basalts. Alkaline OIBs have 3 to perhaps 12-14 %, no ferro basalts are found in alkaline rocks. Iron is less again in arc andesites. There is no built up to a titanomagnetite point as few arc rocks are crystal fractionated, they are mainly partial melts of MORBs and iron decreases from basaltic andesite to rhyolite. Gallium Ga has at times driven me to near despair. It seemed an obvious metal to determine by XRF only there was no gallium standard, so I bought some Ga metal to make artificial standards. Unfortunately it melts at about body heat, so these lumps of what appeared to be aluminum melted whenever touched and ran about like mercury. The obvious solution was to reduce it to some salt that did not melt to we put it in various strengths of HCL (to form GaCl3). It did not dissolve as Al would have done. When boiled it glowed like mercury and slowly diminished in size. After prolonged boiling it vanished. When the acid cooled it reappeared as an immiscible liquid globule quite unchanged. It seemed to be quite indestructible. I finally hurled it out a window and luckily some Ga values appeared for the standard W-1. Uses of Ga GaAs is used in LED's, photocells, laser diodes and IC's and in semiconductors in cell phones. It is anticipated that the use of Ga as a white LED may be billion-dollar industry within a decade. There seem to be no figures for world consumption but various reports mention 15 - 25 tonnes for USA with prices variously given as $425/Kg, $1000/Kg and $3,100/Kg. I smile at reports of Ga being an alternative to Hg in thermometers! Presumably in countries such as Saudi Arabia, daytime use only!!!! Possibly a way has been found of creating an alloy of lower melting point. Ga in Oceanic (Simatic) Crust and OIB's As for certain other elements, Ga has often been left out of analytical data sets, and there are no data for some key associations and islands. Ga in Sialic Rocks Island arc rocks show a lower Ga level but again slope upwards with fractionation. In the South Sandwich Islands of the Scotia Arc we see 12 - 18 ppm and exactly the same in Deception Id where a complex distribution curve is seen, mirrored exactly by Al2O3. We have not yet seen Ga data for clays, zeolites, feldspathoids or other high Al minerals. We can conclude that while Ga often does follow Al closely, the curious way it always increases with fractionation in oceanic rocks while Al increases in alkaline rocks but decreases in tholeiitic ones needs explanation. Germanium At.No. 32, Ge is again produced as a by-product of Zn ore processing and it is also found in coal. The unit price is $1150/Kgm. Ge is a semiconductor and Ge transistors invented by Shockley were the basis of the whole computer industry. By adding dopants of As, Ga, In, Sb and P; Ge could transmit both +ve and -ve currents (or not.) The total consumption is now 110 metric tonnes, with 28,000 kgm being used in the USA according to USGS figures. It has of course been largely supplanted by silicon as a semiconductor. Hafnium Because of the lanthanide contraction Hf has almost identical ionic radius (71 cf 72A) and charge to Zr and never forms its own minerals. It has twice the specific gravity of zirconium and finds special use in atomic power plants. Zircons or baddleyites are usually found to have about 2% Hf. Older data (more than 5 years) often shows more scatter and a wider range of Zr/Hf ratios, sometimes as low as 32 and as high as 50. The newer data shows a concentration about 42 for most rocks. Some of the more reliable data appear to be:
It rather appears that the range of Zr/Hf is from about 37 in depleted MORBs, about 40 in OIB's to 42-45 in alkaline series. Sialic series seem to show a lesser range, the proto-arc andesites being above 40. Indium At. No 49. In is a quite rare element obtained again as a by product of Zn-Pb smelting. About 4 mill. Troy Oz are produce a year at currently $120/oz of which Canada produces 1 million oz. It is used in transistors, rectifiers, photoconductors and LED's. Lithium Li (atomic No 3) is the lightest solid element with a density half that of water. It has a high electropotential and is hence used in batteries. The two main minerals in which it occurs are the lithium mica lepidolite, and as spodumene (LiAlSi2O6), a pyroxene found in pegmatites, but well brines are also a major source. Annual world production is currently 189,300 tonnes of ore at $4,233/t. LiCO3 is used as a flux in glass, ceramics and in alumina smelting potlines. We use it as a flux to reduce melting temperatures of rock powders for XRF analysis but it's use in batteries is rapidly expanding. OIB sodic ankaramite-phonolites show an average range 5.4-45.4 ppm Li with potassic OIB the same. Good Li data has only become available in recent years and is absent in most data sets. Manganese Mn (At.No 25) is an element of many surprises. While totally insignificant of itself, it is the fourth most important metal in industry with a world production which has recently gone up to 25 mt, though there is some doubt as to whether pure metal or ore concentrate is referred to in metals reports. A total value of $2.3 billion US, suggests an average price of $93/t. The greater part of this goes to alloy steels and aluminium, manganese steel being harder, more wear resistant and tougher than mild steel. It is also mentioned in connection with stainless steels but while personally I am familiar with many variants of stainless from type 18-8, type 304, type 302 etc I do not recall a Mn stainless. South Africa has reserves of 12 billion tons, mainly in the Kalahari desert, this being 80% of the world's total estimated reserves. China, the world's greatest steel producer, mines nearly 2 mt annually, some of which is exported while the US imports over half a mt. About 1% of Mn goes into animal feed stock where it forms an essential component. Rhodonite MnSiO3 is an important gangue mineral at Broken Hill and is associated with the Mn olivine and the manganese pyroxene bustamite (CaMnSi2O6). However these are not currently economic to use as ore, in fact some years ago NBH were running the rhodonite back down underground as fill! Anyone want to bet that before too many years are up they will be digging it out againa?? In the oxidation zone Mn occurs as coronadite a hydrated mineral of Pb & Mn. Pyrolusite, MnO2, is found under highly oxidising conditions in bogs or as a secondary product deposited by circulating meteororic waters in Mn-bearing rocks, eg, old volcanic basaltic pyroclastics. Any such rocks stained black or brown with a bluish or purple sheen can be assumed to have a manganese coating. Manganese in Volcanic Rocks In basalts, MnO2 occurs monotonously near 0.18%, varying slightly with amount of iron. It peaks at about 0.3% in ferrobasalts and like Fe and Ti declines rapidly towards rhyolites or trachytes. MORBs have a ratio FeOT/MnO of about 55 and this does not vary greatly. However, in the Macquarie partial melt series MnO increase more rapidly than FeO with decreasing degree of melt, from 0.07 - 0.17% so that MnO should be high in fractionated EMORB. In andesite series a range of 0.4 to 0.2% MnO is typical. In rare instances, Mn can be useful geochemically, eg the Surtsey - Eldfell series can be separated easily on their Mg0/MnO distribution, better than by comparing their MgO/FeO. Magnesium Mg (At. #12) is usually about the 4th most abundant element in basic silicate rocks after O, Si, Al, and sometimes also after Ca and Fe. It is the most important element in classifying igneous rocks geochemically. An MgO level >30% indicates an ultrabasic or peridotitic rock, 12 - 24% a picrite or ankaramite, 6 - 10% a basalt, 3-4% an andesite or mugearite, <1% A trachyte, phonolite or rhyolite. That is, it is affected very strongly by fractionation of igneous rock, being, after Cr, the first element to form a silicate mineral on cooling of a magma. This mineral is usually olivine but may be orthopyroxene in a magma of over 52% silica, followed quickly by clinopyroxene, (plagioclase) and pigeonite. In tholeiitic rocks of the oceanic type, the three minerals olivine, clinopyroxene and plagioclase usually appear together at a cotectic point. Whatever the case, the result is a quick depletion in the amount of magnesium along with Ni, Cr and Co. The site in the common ferromagnesian minerals is increasing taken up by ferrous iron as magmatic temperature declines. Occurrence Though MgO may constitute up to 50% of the mineral olivine and also in ultrabasic rocks which are virtually monomineralic olivine (such as in dunites), separation of metallic MgO from such a high temperature refractory mineral is expensive. Ores are usually serpentenites, and the various halides, carbonates, or nitrates found in salinas, well brines or even seawater. Dolomite,((Ca,Mg),CO3) magnesite, (MgCO3) and brucite, (Mg(OH)2 are also used as a source of magnesium metal. The reserves of magnesium are almost unlimited. Uses Powdered Mg metal ignites at a low temperature and was used extensively as a flare in wartime as it burns with a very intense white flame. A century ago it was used for night photography. It is now used as a lightweight alloy with Al especially in aircraft manufacture and car cylinder blocks and other components. Production Estimates vary widely, an annual production, well below consumption, of 83,000 tons in USA is given by the US Commodities group. Another source gives worldwide production of 730,000 tons with a bulk price of $1.35/lb or $2000/ton. One estimate of the cost of production is $0.60/lb. MgO in Oceanic Basalts Ultrabasic rocks and picrites are quite rare in the oceanic environment, though peridotites do occur as apparent up-faulted slices of mantle, e.g. in St Paul's rocks in the mid-Atlantic and on the walls of some mid-oceanic scarps. 90% of all MORB glasses analysed lie between 6 and 10% MgO with the quite rare associated ferrobasalt-icelandite-dacite-rhyolite rocks decreasing to 1% MgO or less. The average for all MORB rocks is 7.5%. (Note that the latest summary of Melson et al, (2002, G-Cubed) gives a mean of 7.29% MgO for 9050 MORB glasses, no glass sample having in excess of 10%). In Archaean MORB formations however, picrites and rocks with 25 - 35% MgO termed komatiites, appear more common than in their recent equivalents. Ankaramites (an approx. equal mixture of olivine and clinopyroxene) are found in almost all alkali basalt-basanite oceanic islands and sea mounts and may have up to 18% MgO. While a great effort has been made to restrict the analysis of MORBs to non-crystalline glasses, unfortunately no such effort has been made for the alkaline rocks. We therefore have no knowledge what the most magnesian alkali basalt or basanite might be. We therefore do not know where the plane of partial melts lie for alkaline rocks. It seems to be more magnesian than as seen in tholeiites, possibly as high as 12-14%. MgO in Sialic Andesites Proto-arc rocks may have the same MgO content as MORBs, i.e. up to 10%, but in mature-arc or Andean basalt it is rare to fine a non-cumulate rocks with more than 7%, the average basaltic andesite-andesite series averaged 3-5% and in rhyolitic or ignimbritic terrains it is of course usually < 1%. The most basic, depleted rocks known are the so-called "Back-Arc Basin basalts" e.g. of the Lau Basin, (Pearce,J., 1995) but these again do not have more than 10% MgO. MgO in Continental Flood Basalts These vary widely from the picritic rocks of the Letaba Fm in the Karoo, or similar rocks in the Etendeka and the Skaergaard intrusion, where chilled rocks have at least 10%. More commonly the flood "basalts" are LILE enriched and lie between basalt and andesite (50-57% silica) and the magnesium is correspondingly low, usually 3 - 6%. Nickel The metal Ni and to a lesser extent, Co, Zn and Mn all substitute for Mg or Fe in olivine, one of the very first minerals to form in most basalts. Cr also shows much the same behaviour. Under surface conditions Ni preferentially enters olivine to the degree that, (taking an example from the 1959-60 Kilauea Iki picritic eruption, (Gunn, 1971, Chem.Geol. 8, 1-13)), in a melt with about 225 ppm Ni and 7.5% MgO an olivine of Fo85 - Fo88 may have 2500 - 3000 ppm olivine. In other words the partition coefficient is high, perhaps about 13 compared to about 7.5 for MgO. That is, thirteen times as much Ni goes into the olivine as remains in the basalt liquid. A table for partition coefficients for these metals is being prepared.
Origins and Production of Ni
By far the greater part of the Ni in the Earth's crust occurs in the magnesian silicate olivine where it replaces Mg to the extent of 2000 - 4000 ppm. While this only a few tenths of a percent, olivine is a very common mineral in basic rocks. The Ni content is much higher in the more magnesian olivines, ranging from about 3500 ppm at Fo90 down to about 1500 ppm at Fo65. Renner et al (1993,J.Pet.35,361-400) show a much steeper distribution of Ni in olivines of Archaean Komatiites but it is impossible to reconcile their diagram with their data, and we must await new laser ablation ICPMS data. Uses of Ni Nickel is mainly used in stainless alloys for household and hospital utensils, an alloy nickel aluminide is six times as strong as the stainless alloy type 601 (or 18-8), and can withstand temperatures of 1000deg C and is used in gas turbine blades. A Cu-Ni alloy, monel, withstands both seawater and diesel oil corrosion and has wide marine use. Monel is also used in coins. Type 18-8 (18% Cr, 8% Ni) stainless steel is universally used in yacht and other marine fittings Ni in MORBs & EMORBs EPR MORB glasses have a maximum of about 80 ppm which give an approximation
for the most primitive melts, there being no data for Kolbeinsey Ridge,
apparently poor data for Site 504 and no Ni data for the Smithsonian
Deep Sea glasses. The SEIR mainly cluster at 80 - 100 ppm but with a
few up to 200ppm but we are not positive these are all glasses. The
picrites of the south Atlantic studied by Van Heerden and le Roex are
associated with basalts of 80 ppm, as are the picrites of Leg 37 in
the north Atlantic, Gunn & Roobol, 1972). Macquarie Parental melts
show limits of 80 - 160 ppm which explains why basaltic glasses to not
show < 80.
Ni in OIB and MORB picrites Picrites, or olivine-bearing rocks of more than 12% MgO are none so rife hereabouts. Many rocks termed picrite prove to have some cumulative olivine mixed with clinopyroxene and very erratic compositions. True picrites are found in Hawaii, Reunion Island, at one outcrop on the Galapagos Islands at Cerro Redonda, and rarely in mid-ocean ridges but we had some in Site 332B on Leg-37 and Van Heerden and le Roex reported picrites from the South Atlantic (1988, CMPet.100).
Ni in Alkaline OIBs Much to my own surprise, (as I have never really looked at then seriously) the Mg/Ni of some alkaline series cumulates are not greatly different to the tholeiitic rocks in spite of the fact that their cumulates are not pure olivine but about equal amounts of olivine + Cpx, which latter takes in much more Ni. So one would expect about half the Ni in alkaline rocks and about 2/3 the MgO as found in tholeiites.
Ni in Andesite Series The general impression that Ni is very low in andesite series is mainly the result of the scarcity of rocks of 7% or more MgO in andesite series. Most andesites lie in the sub-4% MgO range where the Ni of any rock is low. However it is a pleasure to note than the Morne Caraiibes basalts on Guadaloupe in the Lesser Antilles ARE low in Ni, about 15 ppm at 6% MgO, as we once reported them. The MgO/Ni curve is flatter than for alkali basalts or so it seems from the very few examples we have of high Mg orogenic basalts. Even rocks reported as basalts, eg Hickey, Frey et al, (1986, JGR B91) in south Central Chile, average 6% MgO and 50ppm Ni. Niobium-Tantalum Nb is of great interest to the geochemist because of its sensitive response both to the degree of melt in MORB - EMORB and to the alkalinity of a series, and to the fact of its extreme depletion seen in both orogenic rocks and in Continental Flood Basalts. Y, Zr and Nb, (elements 39, 40, 41) almost always show good correlations as do Y/Lu, Zr/Hf and Nb/Ta.
Sources. Nb-Ta in MORBs and OIBs Nb is extremely low in NMORBS, being commonly in the range 0.1 to 5ppm, (Niu & Batista, 1997). In the EPR series (Regelous et al, 1999) the range is 4-17.6 ppm in basalts-MORB dacites. In the most enriched, low melt fraction, unfractionated EMORBS, Nb can reach over 100ppm, (Kamenetsky, et al, 2000, J.Pet. 41, (3) 411-450).
Nb-Ta in Sialic rocks These are universally anomalously low in abundance in andesitic rocks, nor do they vary much with andesite type. Puyehue and associated centres in south-central Chile have 6 ppm at 60% silica, and the depleted Candlemas Island in the South-Sandwich group about the same. Deception, (as with Zr) is again high at 11 ppm whereas Sangay from Colombia has only 8. Nb is relatively low in the potassic Mt Vesuvius at 20-30 ppm but the Campanian Ignimbrite sweeps up steeply to 120 (Civetta et al, 1997) Ratio Nb/Ta Along with Zr/Hf, the ratio Nb/Ta has long been known to be almost invariant. Like Zr/Hf however it shows a slight change as rocks progress in composition from tholeiitic to alkaline:
The figure for the Macquarie partial-melt series may be dominated by the high Nb-Ta members as the depleted members should not differ from the EPR. Copyright © Dr B.M.Gunn 1998-2006 |